The role of fat and muscles in aesthetic appearance
The majority of human body composition comprises of fat (approx. 25%)1 and muscle (42% male/36% female)2 tissues. Many procedures address subcutaneous fat which correlates with the overall body shape, and its reduction can deliver a slimmer look. The underlying muscles are however equally important as an increased muscle tone better defines the body contour by reducing the localized prolapse/laxity and adds to a healthier aesthetic appearance.
HIFEM® technology
EMSCULPT is based on High-Intensity Focused Electro-Magnetic (HIFEM) field technology which has the ability to induce supramaximal muscle contractions. The rapidly changing magnetic field induces electric currents in the tissue where it depolarizes neural membranes and governs motor units in the target muscles, causing concentric contractions.3,4 The effects are highly selective; due to its physiological characteristics only motor neurons are activated, while other neurons or tissues are not responsive to the current, and therefore stay unaffected.
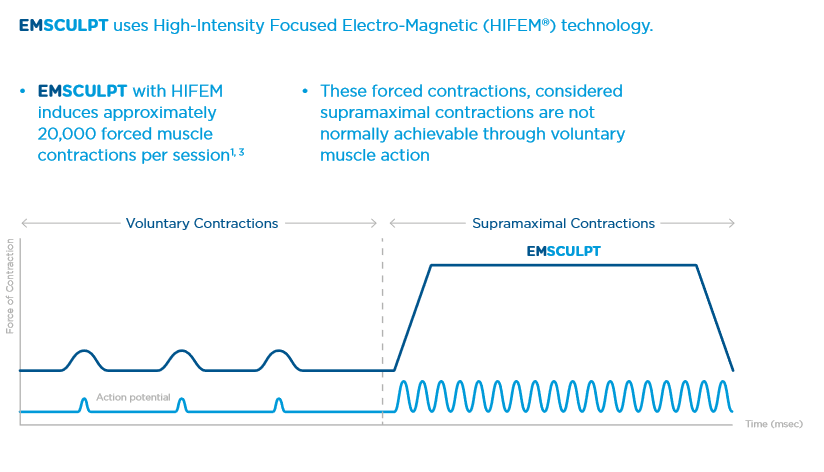
Nerve depolarization for induction of supramaximal contractions
During normal voluntary muscle contractions, the muscle fibers relax between each nervous stimulus due to the central nervous system’s inability to signal another impulse while the previous one is still in action. EMSCULPT generates impulses that are independent of brain function, and at such a rapid frequency that doesn’t allow such a relaxation phase. Under normal conditions, the highest amount of tension that could be developed and held physiologically is called maximal voluntary contraction (MVC). Usually, it lasts only for a split second. Contractions with a tension higher than MVC are defined as supramaximal. EMSCULPT has the ability to generate supramaximal contractions and hold them for multiple seconds, which significantly increases the physiologic stress/workload needed to allow muscles to adapt.
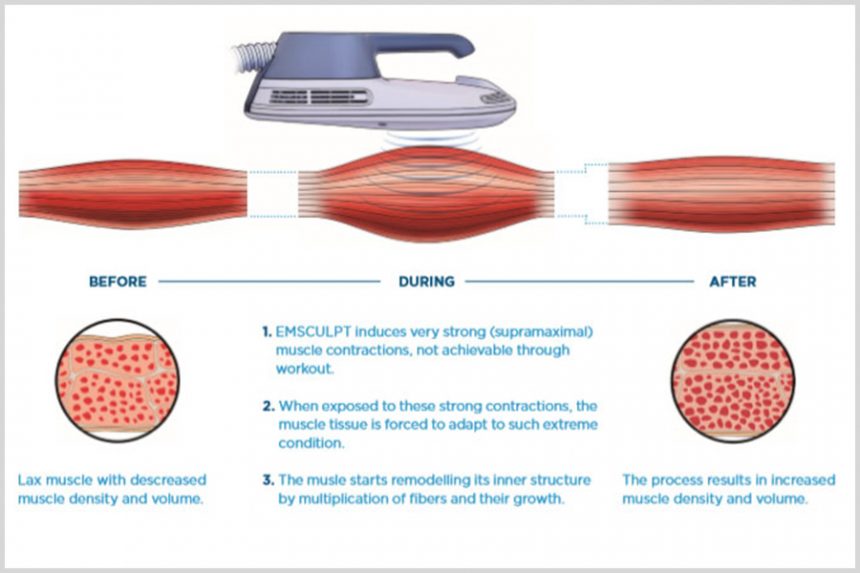
The effects on muscle tissue
When exposed to supramaximal contractions, the muscle tissue is forced to adapt to such extreme conditions and responds with a deep remodeling of its inner structure, i.e., the growth of myofibrils (muscle hypertrophy) and creation of new protein strands and muscle fibers (muscle hyperplasia).5–7 Increased muscle density and volume lead to a better definition and muscle tone. released molecules usually act as an energy source for the needed muscle activity and body metabolism. During an EMSCULPT treatment, the muscles are contracted to supramaximal levels. Signals are sent to the brain that an extreme amount of energy is going to be needed to supply these contractions and the release of epinephrine is acutely increased. This results in an extreme catabolic reaction, and supramaximal lipolysis, which in turn brings about a dramatic release of FFA. When the amount of released FFA exceed normal levels, they start accumulating intracellularly in surrounding adipocytes (see Figure 3) and eventually lead to their dysfunction.9, 12–14 This catabolic and supramaximal lipolysis effect occurs mostly in the area in close proximity to the actual contracting muscles, due to increased adipose tissue blood flow and paracrine substances released from the contracting muscles.9 This principle of cell apoptosis induced by overflow of FFA has been previously observed and demonstrated in numerous research studies.15–17
The clinical effects
What a patient sees after a series of EMSCULPT treatments is a significant growth of their muscles and a reduction of fat. This combination effect on both tissue layers delivers unique aesthetic improvement.
References: 1. Muth ND. What are the guidelines for percentage of body fat loss. Am Counc Exerc ACE Ask Expert Blog. 2009. 2. Marieb EN, Hoehn K. Human Anatomy & Physiology, 8th Edition. 8th edition. San Francisco: Benjamin Cummings; 2009. 3. Wallis MC, Davies EA, Thalib L, Griffiths S. Pelvic Static Magnetic Stimulation to Control Urinary Incontinence in Older Women: A Randomized Controlled Trial. Clin Med Res. 2012;10(1):7-14. doi:10.3121/cmr.2011.1008 4. Yokoyama T, Fujita O, Nishiguchi J, et al. Extracorporeal magnetic innervation treatment for urinary incontinence. Int J Urol. 2004;11(8):602-606. doi: 10.1111/j.1442-2042.2004.00857.x 5. Ostrovidov S, Hosseini V, Ahadian S, et al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng Part B Rev. 2014;20(5): 403-436. doi:10.1089/ten.TEB.2013.0534 6. Coletti D, Teodori L, Albertini MC, et al. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytom Part J Int Soc Anal Cytol. 2007;71(10):846-856. doi:10.1002/cyto.a.20447 7. Abulhasan JF, Rumble YLD, Morgan ER, Slatter WH, Grey MJ. Peripheral Electrical and Magnetic Stimulation to Augment Resistance Training. J Funct Morphol Kinesiol. 2016;1(3):328-342. doi:10.3390/jfmk1030328 8. Alsted TJ, Ploug T, Prats C, et al. Contraction-induced lipolysis is not impaired by inhibition of hormone-sensitive lipase in skeletal muscle. J Physiol. 2013;591(20):5141-5155. doi:10.1113/jphysiol.2013.260794 9. Stallknecht B, Dela F, Helge JW. Are blood flow and lipolysis in subcutaneous adipose tissue influenced by contractions in adjacent muscles in humans? Am J Physiol Endocrinol Metab. 2007;292(2):E394-399. doi:10.1152/ajpendo.00215.2006 10. Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19(5):549-555. doi:10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B 11. Bustamante V, María EL de S, Gorostiza A, Jiménez U, Gáldiz JB. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respir Med. 2010;104(2):237-245. doi:10.1016/j.rmed.2009.10.001 12. Zha BS, Zhou H. ER Stress and Lipid Metabolism in Adipocytes. Biochemistry Research International. doi:10.1155/2012/312943 13. Tripathi YB, Pandey V. Obesity and endoplasmic reticulum (ER) stresses. Front Immunol. 2012;3. doi:10.3389/fimmu.2012.00240 14. Ghosh AK, Garg SK, Mau T, O’Brien M, Liu J, Yung R. Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci. 2015;70(11):1320-1329. doi:10.1093/gerona/glu186 15. Gunduz F, Aboulnasr FM, Chandra PK, et al. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol J. 2012;9:143. doi:10.1186/1743-422X-9-143 16. Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293(2):E576-586. doi:10.1152/ajpendo.00523.2006 17. Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi:10.1186/1476-511X-11-1